The US Meals and Drug Administration has authorised two injectable variations of the blockbuster weight-loss and diabetes drug, semaglutide (Wegovy and Ozempic). Each are available pre-filled pens with pre-set doses, clear directions, and details about overdoses. However, given the medicine’ daunting costs and provide shortages, many sufferers are turning to imitations—and people do not at all times include the identical security guardrails.
In an alert Friday, the FDA warned that individuals are overdosing on off-brand injections of semaglutide, that are allotted from compounding pharmacies in quite a lot of concentrations, labeled with varied models of measurement, administered with improperly sized syringes, and prescribed with unhealthy dosage math. The errors are main some sufferers to take as much as 20 instances the quantity of supposed semaglutide, the FDA experiences.
Although the company would not provide a tally of overdose instances which were reported, it suggests it has acquired a number of experiences of individuals sickened by dosing errors, with some requiring hospitalizations. Semaglutide overdoses trigger nausea, vomiting, belly ache, fainting, headache, migraine, dehydration, acute pancreatitis, and gallstones, the company experiences.
Dangerous math
In typical conditions, compounding pharmacies present customized formulations of FDA-approved medicine, as an example, if a affected person is allergic to a particular ingredient, requires a particular dosage, or wants a liquid model of a drug as a substitute of a tablet type. However, when commercially accessible medicine are in brief provide—as semaglutide medicine at present are—then compound pharmacies can legally step in to make their very own variations if sure situations are met. Nevertheless, these imitations are usually not FDA-approved and, as such, do not include the identical security, high quality, and effectiveness assurances as authorised medicine.
Within the warning Friday, the FDA mentioned that some sufferers acquired complicated directions from compounding pharmacies, which indicated they inject themselves with a sure variety of “models” of semaglutide—the quantity of which can range relying on the focus—slightly than milligrams or milliliters. In different situations, sufferers acquired U-100 (1-milliliter) syringes to manage 0.05-milliliter doses of the drug, or 5 models. The comparatively massive syringe measurement in contrast with the dose led some sufferers to manage 50 models as a substitute of 5.
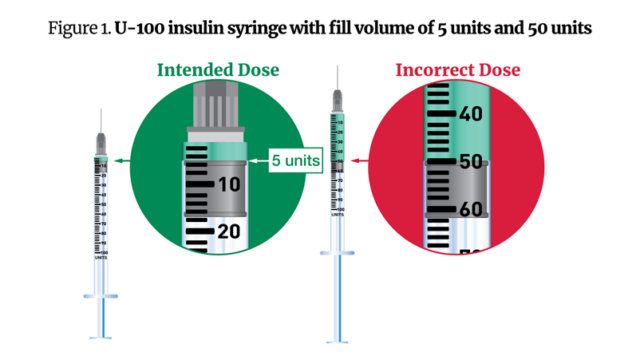
FDA-approved semaglutide medicine, in the meantime, are dosed in milligrams and are available standardized concentrations. The company acquired a number of experiences of well being care suppliers incorrectly changing from milligrams to models or milliliters, main them to calculate the incorrect dosages. With these math errors, some sufferers administered 5 to 10 instances extra semaglutide than supposed.
“FDA acknowledges the substantial shopper curiosity in utilizing compounded semaglutide merchandise for weight reduction,” the company wrote. “Nevertheless, compounded medicine pose a better threat to sufferers than FDA-approved medicine.” The company urged sufferers and prescribers to solely use compounded variations when completely crucial.